OUR PLATFORM
Our focus at Hoba Therapeutics is the development of novel therapeutics for the treatment of neuropathic pain and hearing disorders.
Hoba Therapeutics holds the rights to two novel biopharmaceutical compounds –
HB-086 and HB-097, currently in development for the treatment of chronic pain disorders and hearing loss, respectively.
HB-086 and HB-097 are therapeutic proteins and the only members of a family of recently discovered neurotrophic factors. Our research confirms these compounds have unique actions on sensory nerve cells and their surrounding support cells.
HB-086
HB-086 is recombinant human meteorin. HB-086 has shown analgesic and long-lasting relief in multiple models of chronic neuropathic pain through a novel mechanism of action. Meteorin is known to have several functions, including inducing and directing neurite outgrowth1, neuroprotection2, and myelination3.
HB-086 is a non-opioid treatment targeting the peripheral nervous system.
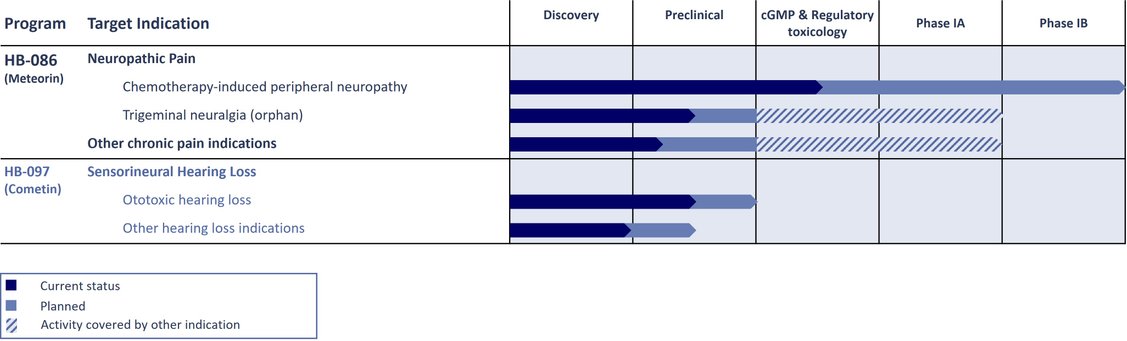
HB-086 is in late preclinical development.
1) Promotion of the growth of neurites, which are the long, thread-like extensions of nerve cells. This function is important for the development and maintenance of a healthy nervous system.
2) Protection of the nerve cells from damage and promoting their survival under certain conditions, which can be important in various neurological disorders.
3) Myelination is the process by which nerve cells are insulated with a fatty substance called myelin, which speeds up the transmission of nerve signals. Meteorin has been linked to the regulation of myelination in the nervous system.
HB-097
HB-097 is a recombinant human cometin. Hb-097 has shown promising therapeutic and protective properties in models of hearing loss.
HB-097 is in preclinical development.
OUR PIPELINE